By now, if you're still treating silver as some peripheral add-on in lithium battery design, you haven’t read the damn literature. The recent lab work isn’t subtle: silver—specifically in nanostructured form—is fixing problems that engineers have been brute-forcing for a decade.
This isn’t about making batteries 2% better. It’s about getting rid of dendrites, stabilizing cathode cycling, and building solid-state lithium packs that actually work under mechanical stress. Silver isn’t the garnish. It’s the glue.
Let’s Start With the One Everyone Should Be Talking About:
A Flexible All-Solid-State Li Battery Using Ag–Li Anodes
The setup is deceptively simple:
- Anode: Lithium alloyed with silver nanowires
- Cathode: V₂O₅ nanowires bound with carbon nanotubes
- Electrolyte: LLZO-based solid-state layer with CeO₂ microspheres
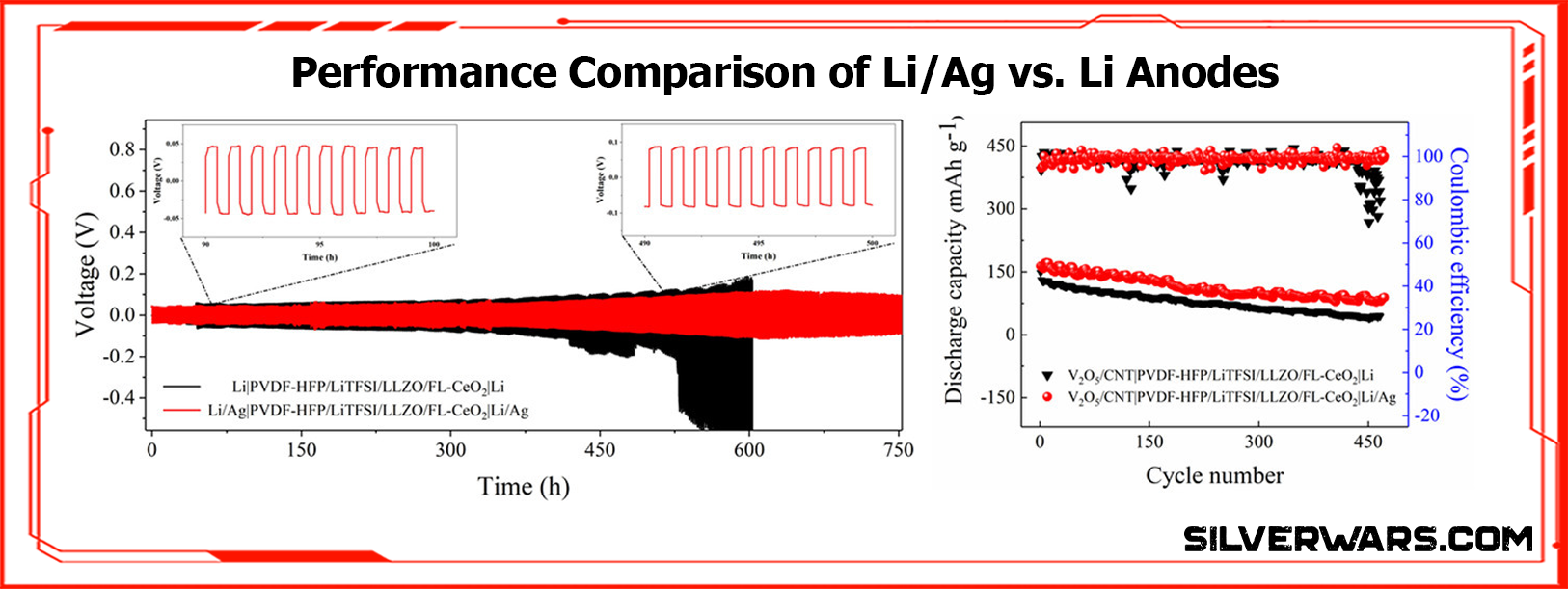
This is not a toy lab prototype. This battery powered a 3V LED display under stress conditions—bent, flexed, under load. What’s more, it didn’t die after 100 cycles like most of these overhyped “flexible” cells do.

Performance Metrics That Actually Matter
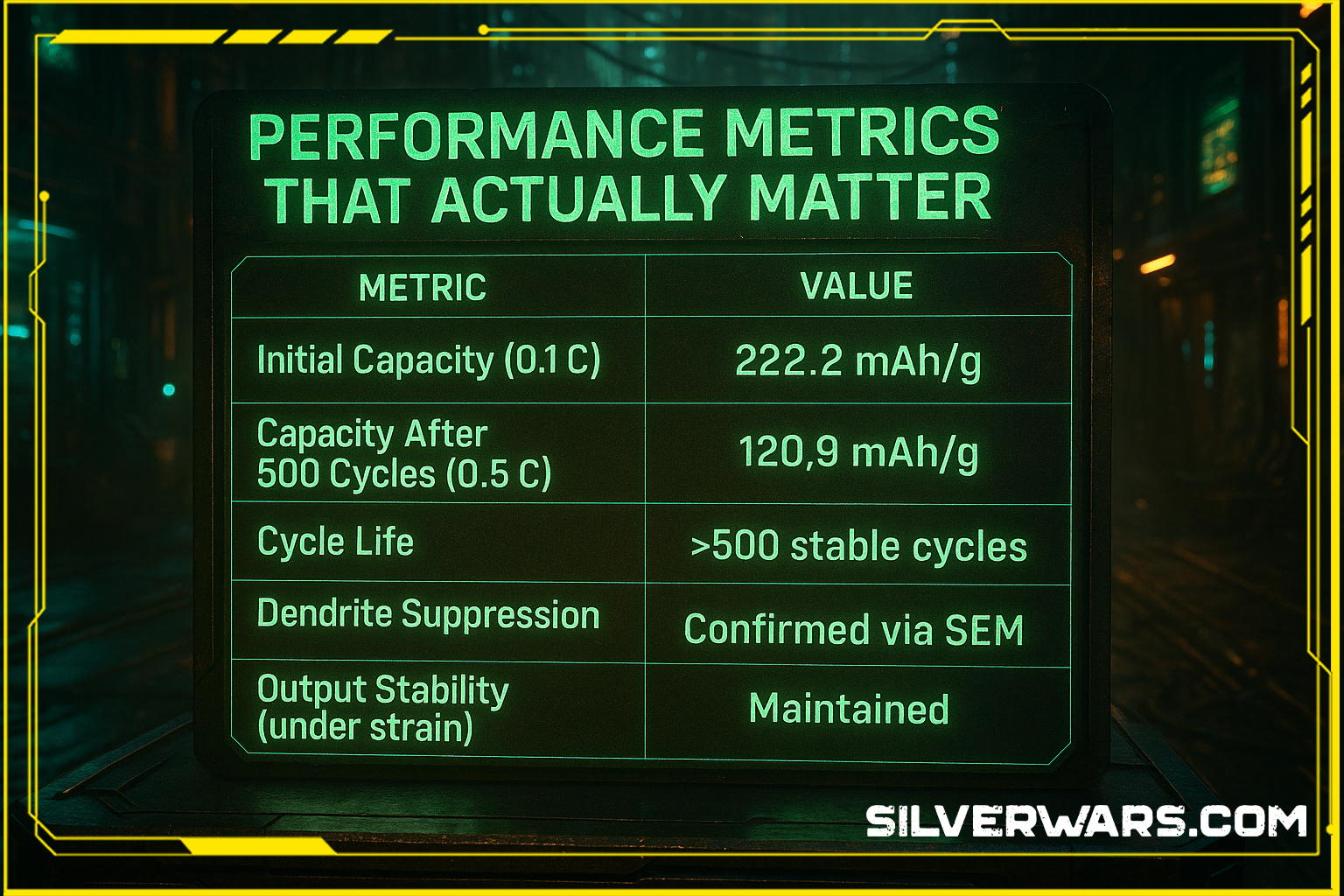
| Metric | Value |
|---|---|
| Initial Capacity (0.1 C) | 222.2 mAh/g |
| Capacity After 500 Cycles (0.5 C) | 120.9 mAh/g |
| Cycle Life | >500 stable cycles |
| Dendrite Suppression | Confirmed via SEM |
| Output Stability (under strain) | Maintained |
Now look me in the eye and tell me silver’s role is “minor.”
Looking to diversify your portfolio with tangible assets? Jim Cook at Investment Rarities offers expertly curated asset investments with their extremely dedicated team. Discover unique opportunities often overlooked by traditional markets. Visit investmentrarities.com today!
What’s the Silver Actually Doing?
Let’s be clear: dendrites are the ticking time bomb of every lithium metal battery. You get plating irregularities, tip growth, eventual short circuits, thermal runaway—you know the drill.
But alloying lithium with silver nanowires changes the surface kinetics. It creates a lithium-conductive matrix that doesn’t just delay dendrite growth, it strangles it. What you get is uniform lithium deposition right into the electrode framework. No spikes. No ruptures.
SEM imaging after 500 cycles confirms it—flat surface, no filamentary growth. Try that with a pure Li foil.

Wall Street Silver Reddit is the Home of #SilverSqueeze. We love silver. Every Troy Ounce! No diamond-hands without EXTREME Pressure!
[Now Under SILVER WARS Management]
Meanwhile, Across the Bench—Silver on the Cathode Side
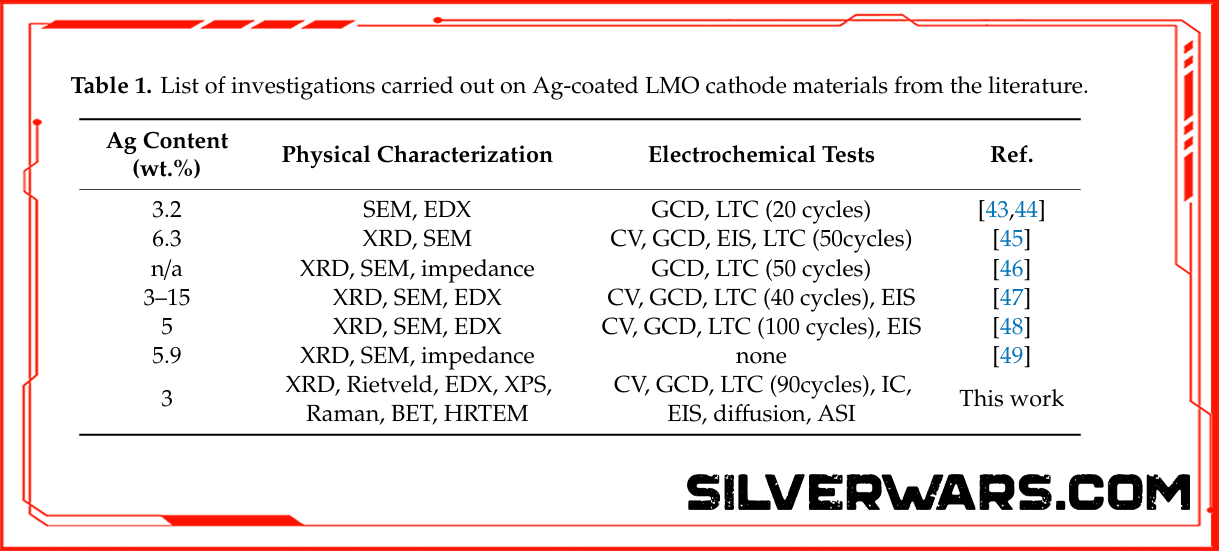
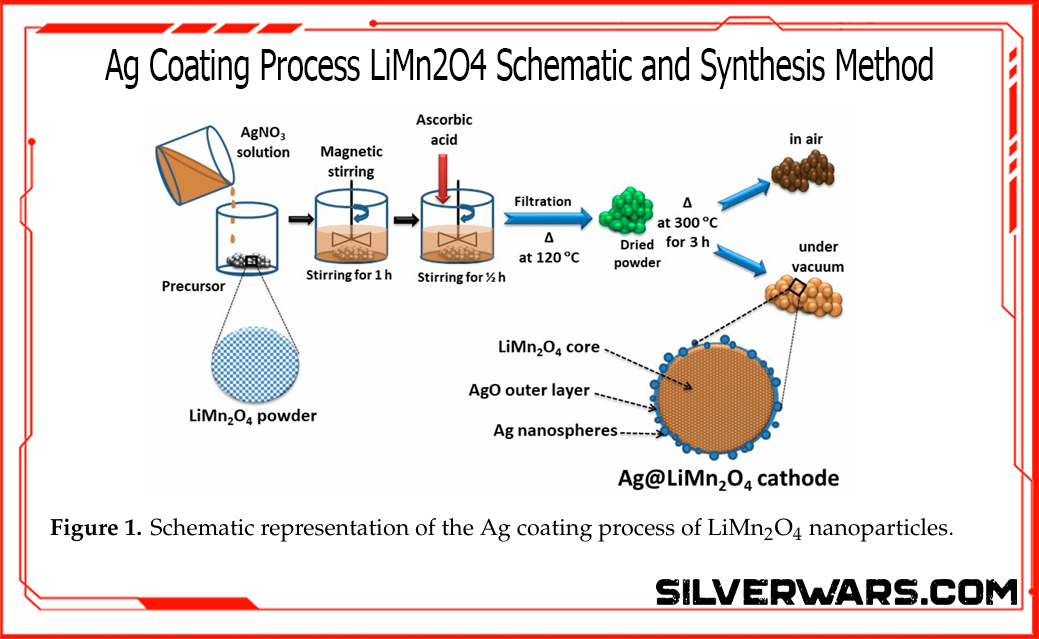
Let’s shift to a second study, where researchers coated LiMn₂O₄ cathodes with ~3 wt.% silver. Two versions were tested:
- Ag/LMO(a): calcined in air (bad idea)
- Ag/LMO(v): calcined in vacuum (preserved Ag⁰ nanospheres)
Only the vacuum-treated sample maintained high conductivity and avoided forming AgO—an electrical dead end.
Silver Wars is dedicated to Safe Guarding the World's Silver Future.
Ag-Coated Cathode Results
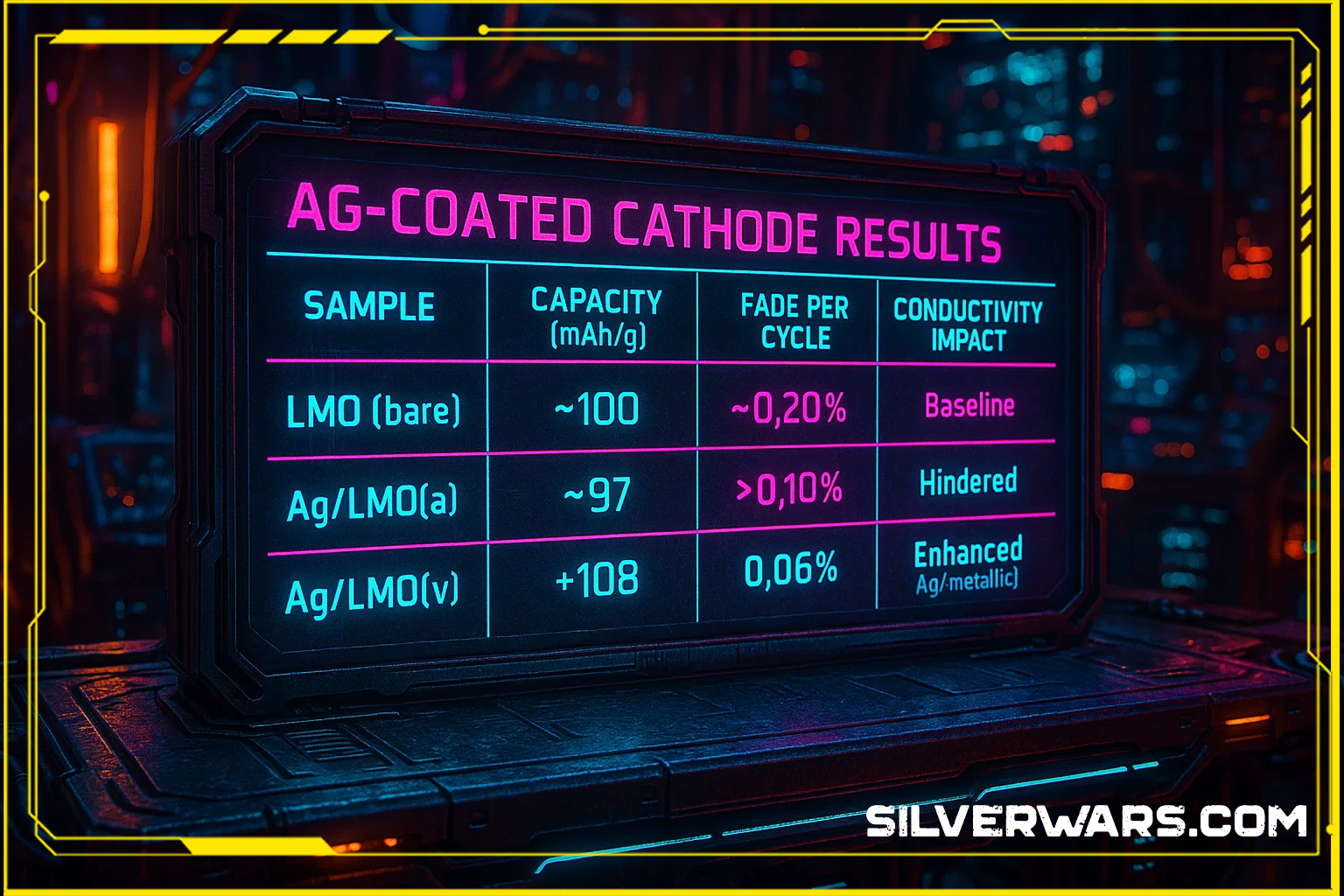
| Sample | Capacity (mAh/g) | Fade per Cycle | Conductivity Impact | Dominant Ag Species |
|---|---|---|---|---|
| LMO (bare) | ~100 | ~0.20% | Baseline | None |
| Ag/LMO(a) | ~97 | >0.10% | Hindered | AgO (insulating) |
| Ag/LMO(v) | ~108 | 0.06% | Enhanced | Ag⁰ (metallic) |
The cathode with metallic Ag survived longer, moved electrons faster, and kept impedance low. Once again: silver isn’t acting as a dopant—it’s acting like a structural conductor and chemical stabilizer. Exactly what you’d want if you were serious about building resilient lithium-ion infrastructure.

Morphology: Here’s What It Actually Looks Like
If you're still debating whether Ag⁰ or AgO dominates these materials, stop guessing and look at the damn microscope.
The Figure below shows the TEM and high-res imaging results for pristine LMO, vacuum-treated Ag/LMO(v), and air-treated Ag/LMO(a). The difference isn’t subtle.
- Pristine LMO (a, b): Clean particles with well-defined 0.47 nm fringes corresponding to the (111) plane of LiMn₂O₄. Nothing fancy, just structurally stable spinel.
- Ag/LMO(v) (c, d): Now we see silver doing its job—uniformly distributed 8–15 nm Ag nanoparticles, confirmed by the 0.239 nm lattice fringes (Ag (111)).
- Ag/LMO(a) (e, f): Total disaster. The surface is coated with oxidized silver—0.295 nm AgO (110)—which does nothing but introduce impedance and ruin the active surface area.
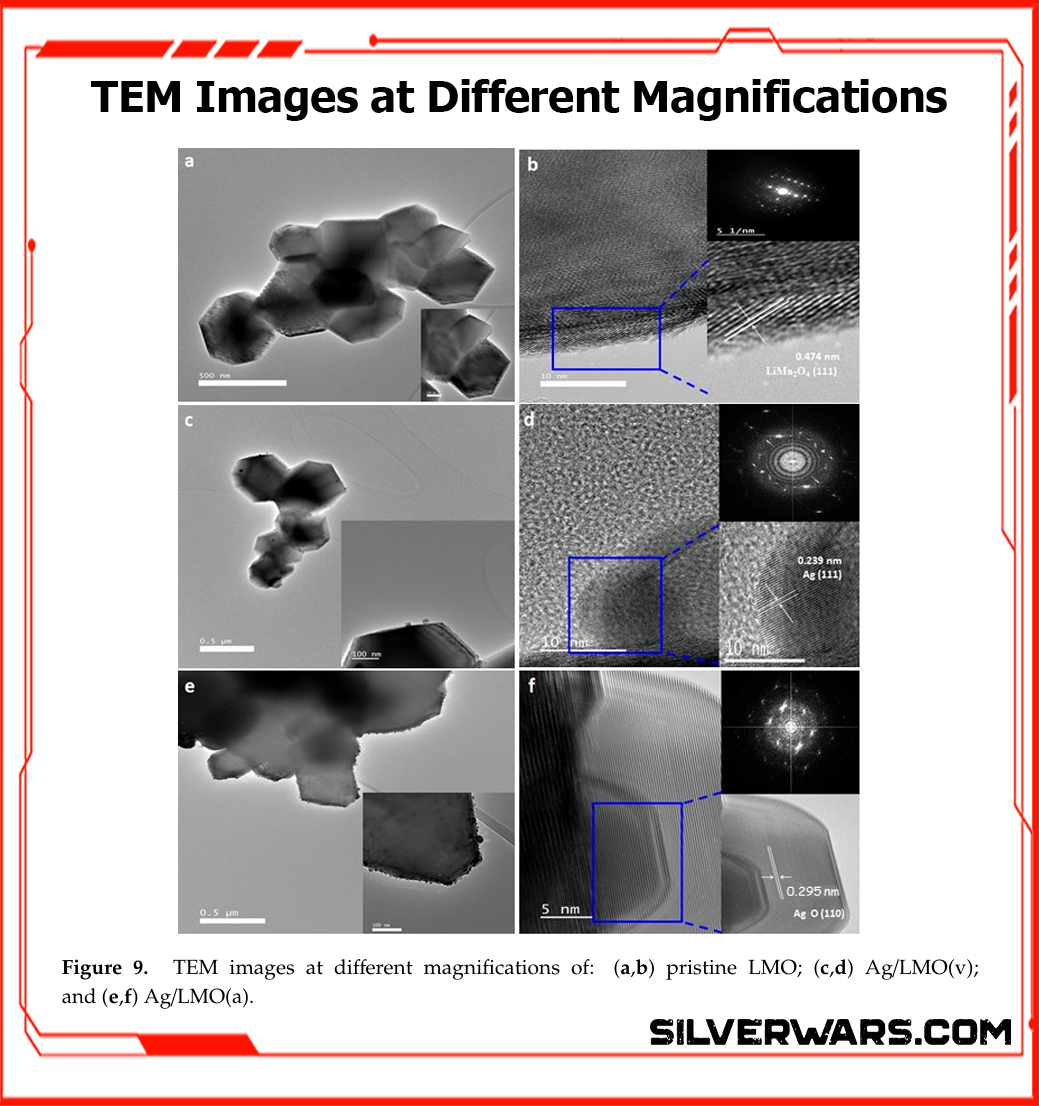
This isn’t theoretical. You can literally see the conductive silver in the vacuum sample and the insulating oxide crust in the air-treated one. That’s the difference between a cathode that fades slowly and one that tanks halfway through the test.

Industry Implications: Stop Treating Silver Like an Afterthought
Battery electrode coating machine like the TOB-JS400-2J is generally designed to coat various types of slurries onto electrode substrates, and that can include slurries containing silver.
People still act like silver use in batteries is niche. That’s either laziness or intentional ignorance. Let’s run some quick numbers:
- Flexible SSDs with Ag–Li anodes: ~100 mg silver per unit
- EV batteries with Ag-coated cathodes: 300 g per pack at 3 wt.%
- Global EV production at 10M/year = 3,000 metric tons of silver
That’s before you count drones, wearables, or military assets using high-cycling solid-state formats. And no, you can’t swap in aluminum or copper and get the same results. We've tested that.

The Silver Wars take Many Shapes...
The data isn’t ambiguous. Whether you’re fixing lithium metal’s dendrite problem or stabilizing LMO cycling behavior, the common denominator is silver—and not in some theoretical capacity. We're talking real mass loadings, real capacity gains, and real suppression of failure modes.
You want to design batteries that last 500+ cycles, don’t catch fire, and survive mechanical deformation? Then stop hand-waving silver away as "too expensive" and start running the numbers on performance per gram.
Silver isn’t a nice-to-have. It’s doing the hard work. And anyone ignoring that is setting their R&D budget on fire.










